Membranes and barriers are used all the time in industrial and lab settings, and you may even have a few of them around the home. They can help keep materials apart that need to be separated, or can selectively allow certain materials to mix while holding others back. Graphene, the two-dimensional hexagonal lattice of carbon, is thought to be completely impermeable to all gases and liquids. That would obviously make it an extremely effective barrier film.
Creating sufficient quantities of pure graphene is not an an easy task, but graphene oxide (GO) films can be readily made in the lab. There's a paper in last week's edition of Science that examines the properties of graphene oxide films.
The authors examined the physical structure of the GO films using scanning electron microscopy and x-ray analysis. They confirmed that the films were layered structures consisting of individual crystallites a few microns in diameter (crystallites are the basic units of a polycrystalline material) with a five angstrom separation. The researchers created membranes of this material that ranged from 0.1 to 10 microns in thickness and measured about one centimeter in diameter. These membranes, even those with a thickness of less then a micron, were reasonably strong, withstanding differential pressures across them of up to 100 mbar.
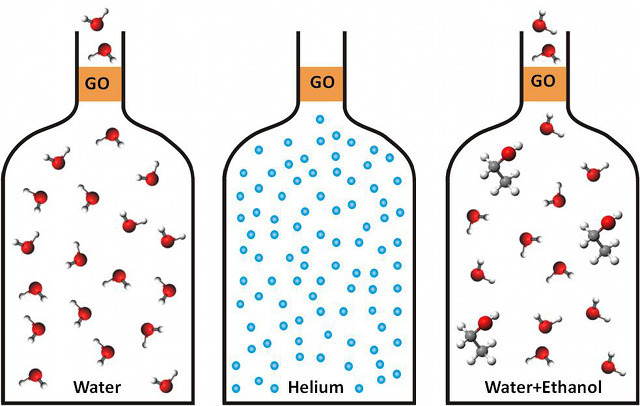
Initial experiments with gases such as helium, hydrogen, nitrogen, and argon found that almost no gas was able to move across the membrane over a period of a few days. Calculations on these results yielded a helium permeation rate below 10-12 g/cm2*s*bar, consistent with values reported elsewhere for pure graphene films—basically no gas was getting through. Computing the bulk permeability of the material gave a value of approximately 10-15mm*g/cm2*s*bar. Put into more useful terms, this means that less gas will seep through a submicron thick GO film than would pass through a 1 mm thick glass barrier in an equivalent amount of time!
Carrying out a similar experiment with common liquids (ethanol, hexane, acetone, decane, and propanol) revealed that no weight loss could be detected after several days of the fluid resting on the membrane. This set an upper limit on liquid bulk permeability of 10-11 mm*g/cm2*s*bar.
However, something unexpected happened when they repeated the test with water. There was a huge weight and the evaporation rate was nearly the same as though there was no membrane or barrier in place.
After this unexpected result, the authors repeated the test with helium to ensure that no physical damage had occurred to the membrane; no helium leakage was observed. In fact, it was only when the membrane was a couple of microns thick that any resistance to the flow of water was observed. Computing the bulk permeability of water gave a result of 10-5 mm*g/cm2*s*bar, a value 1010 (10,000,000,000) times greater than that for helium. This membrane was essentially impermeable to a small, inert gas, but allowed water to freely move through it.
To get a better idea of what was happening, the team carried out permeation experiments using mixtures of water with other gases and liquids. In the presence of saturated water vapor, helium was able to traverse the membrane, albeit five orders of magnitude more slowly than the water. The authors noted that the rate at which helium moved through was actually the same rate at which helium would diffuse through a similar column of water, suggesting that it was diffusing through the freely moving water.
Another series of experiments compared the rate of water permeation through the membrane to the rate at which water evaporated in an open-ended system. By varying the pressure across the membrane and the relative humidity in the test chamber, the researchers determined that water was limited only by the rate of mass transfer at the far surface—the rate of evaporation after the water had moved across the membrane was limiting how quickly it could move through the membrane. There was a molecular traffic jam on the far end holding up the entire process.
To explain how this was possible, the authors hypothesized that the size between the layers of graphene were just right. They suggest that, at a free channel spacing of just about five angstroms, a monolayer of water forms that is capable of undergoing a low-friction flow in the two-dimension channels that exist between the layers (take that, no slip hypothesis!). To move between layers—and hence traverse the membrane—the authors posit that a percolating network of graphene nanocapillaries exists that allows the water to flow throughout and across the membrane.
To test this hypothesis, the authors carried out a handful of experiments. First, they reduced the graphene oxide material by calcining it in a 250oC hydrogen-argon atmosphere. This resulted in a reduction of water permeability by a factor of 100. The authors attribute this to the reduction in the channel width; X-ray examination of the material showed it was reduced to about four angstroms, which is too small for a monolayer of water to form and flow.
To further back up the idea that the high water permeability was due to the just-right size of the channel, the authors ran a series of molecular dynamics simulations that modeled how a reservoir of water molecules would move through a single, two-dimensional graphene pore. By varying the pore width, they showed that water could not form a monolayer and flow in pores smaller than six angstroms. Above 10 angstroms, a second monolayer would form that would greatly reduce the flow. It was the intermediate region that allowed for a low-friction flow of a monolayer of water.
While this represents an interesting result with a wide variety of applications—water removal, water filtration, degassing, and dewetting in industrial settings—an accompanying perspective in the same issue of Science puts the discovery, well, in perspective. Any new industrial use of such discoveries is a long way off, because we first need to learn how to make larger membranes (the ones used in the article are ~1 cm across). They'll also need to stand up to industrial scale pressures, and be packaged into a useful device. While these are important discoveries, their immediate uses will be limited to small scale and niche applications.
Science, 2012. DOI: 10.1126/science.1211694 (Article), 10.1126/science.1216923 (Perspective).
Listing image by Photograph by Science/AAAS
reader comments
65