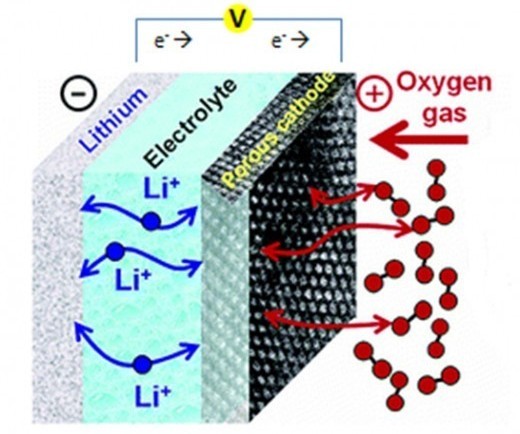
Lithium-air batteries are the coming thing. They have the potential to hold ten times the capacity per unit volume of lithium-ion batteries and are lighter, since one of the charge-carrying materials is ambient air. These air-breathing monsters look great. But, as with all monsters, they have their vulnerable spot. In this case, the vulnerability is something akin to asthma—the inability to get enough air into the battery. Medical scientists don`t seem to be too interested in making inhaler products for batteries, though, so scientists have been trying to come up with ways to keep the air flowing.
In some recent work, published in Nano Letters, researchers show that producing graphene as poorly as possible helps a lot. Yes, it truly is a case of deliberate incompetence saving the day.
Aside from the drama of monsters with asthma, why are we interested in lithium-air batteries anyway? The key is energy storage. You can only pack so many lithium ions into a given space. Given that limit, the amount of energy you get out depends on the reaction potential at the electrodes, which are typically graphite—graphene's poor country cousin. Clearly, a serious change in technology is required if we are to meet the demands of the future: electric cars don't run on air, you know.
In a lithium air battery, one of the electrodes is the lithium itself and its reaction with air takes place at a conducting carbon electrode. But the end product of the reaction is lithium oxide, which isn't soluble. So, lithium turns up at the electrode, reacts with the oxygen in the air, and the departing electrons leave behind a little lithium oxide deposit. Eventually, these deposits clog up the system and the battery dies a slow, gasping death.
To overcome this problem, a team from Pacific Northwest National Laboratory and Princeton have taken an idea from nature. Smokers face the same problem the battery does every day. Every cigarette that they smoke leaves behind some particulate matter that reduces lung function. Yet, smokers generally go for many years before their reduced lung capacity becomes noticeable.
This is because the lung consists of a branching network, where the tiny alveoli—the point where oxygen enters the blood stream—are never very far away from a big tube, one that's too big to be easily blocked. There are lots and lots of alveoli, so you can afford to lose a few, but the big tubes make sure that losing an alveoli due to blockage doesn't block up neighboring alveoli.
The question then is, how do you do this in carbon? It turns out to be not too difficult. Take one lump of graphite oxide and begin to remove the oxygen. As the oxygen is removed at high temperature, the graphite expands into many graphene sheets, tearing itself to pieces to create micrometer sized holes throughout the structure. Each graphene sheet still has a fair bit of oxygen associated with it, which are defects that create nanometer-sized holes in the graphene sheets. The result is the lowest-quality graphene known to man—something to be proud of in itself—and a lovely network of tiny reaction centers, interconnected by big tubes.
The cool thing is that the balance between the large holes that provide the main air tubes and the small holes that play the role of reaction sites can be controlled just by playing with the amount of time spent removing the oxygen from the graphite oxide. The researchers tried a number of different ratios and found that leaving just one percent of the oxygen behind gave the best battery performance.
If that wasn't enough to make you fall in love with the monster, there is another advantage to this electrode. The lithium oxide deposits should still eventually clog things up, but something unexpected—unexpected to me, anyway—happens. The lithium oxide deposits are only stable up to clumps of five units. That is, when a sixth lithium oxide molecule tries to join the party, the clump is likely to fall apart, keeping the lumps small and maintaining open airways.
Finally, the information you have all been waiting for: how much energy can I get from this battery? The researchers demonstrated 15000mAhr per gram of carbon electrode. And that seems pretty fantastic to me.
So, as my kids say to me: are we there yet? Nearly. The capacity measurements that they showed were taken at twice atmospheric pressure, so there is some question about how the batteries would perform at atmospheric pressure and 20 percent oxygen. Then there is the question of recharging. In principle, lithium air batteries are rechargeable, but a metallic catalyst at the carbon electrode is required. In this work, the researchers did not include the catalyst, so no recharging.
Even so, this is the right path to take: this beast no longer gasps for air. With more optimization, metal catalysts can be worked into the mix.
Nano Letters, 2011, DOI: 10.1021/nl203332e
Listing image by Photograph by NASA
reader comments
62