The promise of embryonic stem cells lies in their ability to develop into any type of cell in the human body, which should allow us to replace tissues lost due to injury or disease. But it's one thing to generate replacement cells; it's another thing to generate entire tissues and integrate them into a functioning organ. A paper released by Nature now reports some success with turning human embryonic stem cells (hESCs) into cardiac cells and getting them to beat in synchrony with a damaged heart.
The blockage of blood vessels in the heart, either through clots or occlusion, causes the cells that rely on the blocked vessel to die off. This both weakens the heart structurally and changes the ability of the heart to beat in an organized manner, since the scar tissue that develops doesn't conduct electrical impulses. Serious arrhythmias can develop as a result of this changed activity, and these can sometimes end up causing the heartbeat to be lost entirely.
Embryonic stem cells have been used to try to repair damaged hearts for a while, starting with simple experiments where the stem cells themselves were injected. More recently, researchers have induced hESCs to form cardiac muscle cells (cardiomyocytes) before implanting them in a damaged heart (typically that of a mouse or rat). This treatment tends to increase the ability of the heart to pump blood, indicating that stem cells can reverse the weakening of the heart.
But it has been harder to get at the electrical integration of these stem cells, in part because the rodents that the researchers used have a very fast heartbeat—on the order of 400-600 beats a minute. (The human heart rate is normally under 100 beats per minute.) So, the new work relied on the guinea pig, which apparently has a heart rate that is only about 200-250 beats per minute.
The authors took an hESC line and induced it to form cardiomyocytes, which were injected into injured hearts and then allowed to integrate with the injured heart for a while. Rather than focusing on blood flow, the authors tracked the development of arrhythmias. It turns out that the hESC-derived cardiomyocytes suppressed them. The guinea pigs treated with them had the lowest rate of premature ventricular contractions, or PVCs, which occur when the lower chambers of the heart beat ahead of schedule. They also went into tachycardia, or a run of rapid heartbeats, less often.
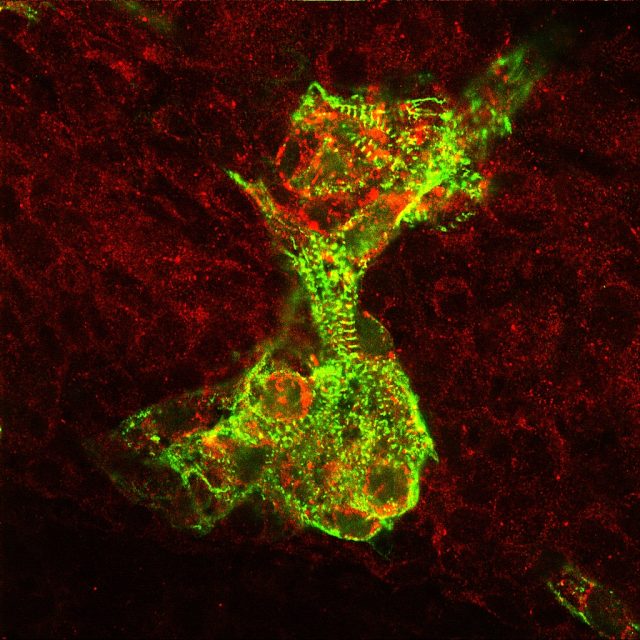
To track the behavior of the hESC-derived cardiac cells, the authors inserted a gene for a protein that becomes fluorescent in response to changes in calcium, which accompany the electrical impulses that drive a heartbeat. By tracking whether a cell was glowing, the authors could determine whether the human cells were tied to the regular guinea pig heartbeat.
Here, the results were a bit mixed. In areas where the hESC-derived cells were stuck in an area with lots of scar tissue, they tended to contract on their own, without significant influence from the guinea pig's rhythm. But in other areas where the cells were clear of nearby scar tissue, they tended to tie in nicely with the heart's overall rhythm—even when they weren't necessarily close to any guinea pig tissue.
The results are very promising, in that they show that embryonic stem cells can be used to create a large population of cardiomyocytes that can then function normally when placed back into a heart. But they also make it clear that scar tissue remains a problem in damaged hearts. Even if muscle tissue gets replaced, it won't integrate well if there's a significant amount of scar tissue around. This provides researchers with an obvious target for future efforts.
Incidentally, a number of the researchers involved in this work were based at US institutions. Early in the history of stem cell research, legislation was considered that would ban the creation of human-animal hybrids. Although it was probably written with Frankenstein-like chimeras in mind, some of it was so broadly worded that it would have banned basic safety and efficacy research such as the work described by this paper. Fortunately, it never passed, so US researchers are still able to contribute to work like this.
Nature, 2012. DOI: 10.1038/nature11317 (About DOIs).
reader comments
22