We'll probably never know exactly how life on Earth got its start. The conditions in which it began have long since been lost, and there are simply too many precursor molecules and potential environments that could have gotten the process going. Nevertheless, researchers hope to put together a pathway that's at least plausible, starting from simple molecules that were present on the early Earth and building up to an enclosed system with basic inheritance (from there, evolution can take over).
A lot of progress has been made in understanding how a simple chemical, like hydrogen cyanide, can be built up through a series of reactions into a nucleotide, the basic building block of molecules like DNA and RNA. And we've learned quite a bit about how larger RNAs (more than 100 nucleotides long) can fold into complex structures that can catalyze reactions and undergo the chemical equivalent of Darwinian evolution. The challenge has been bridging the gap between the two, going from a handful of linked nucleotides to a large molecule that's potentially capable of catalyzing chemical reactions.
Now, the team that developed the earlier results is back with another publication. Their latest work shows how short molecules that are composed of just a handful of nucleotides can be linked together, eventually building longer, more complex chains. Once again, the chemistry is simple enough to occur on the early Earth, and the reaction might explain a curious bias in how DNA and RNA are built into long chains of nucleotides.
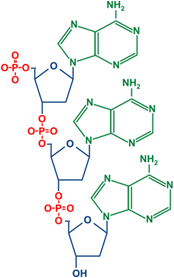
To understand the chemistry, it's critical to understand the structure of DNA and RNA. Both have a linked backbone that alternates between phosphates (in red) and sugars that are formed into a five-atom ring (in blue, with the O on top). In RNA, the two lower corners of the ring (positions 2 and 3, counting clockwise from the O) are chemically very similar in that they both have oxygens hanging off them. In the chemical synthesis described in one of the previous papers, the authors found that the phosphate was linked to both position 2 and 3, instead of only being linked to position 3.
The authors were curious about how the exclusive bias toward position 3 occurred, so they considered the possibility that another chemical reaction could knock the phosphate off position 2. They tested a very simple compound, one containing only two carbons: thioacetate, which can form spontaneously through the reaction of carbon monoxide and hydrogen sulfate. Both of these carbons are expected to be present in the atmosphere of the early Earth.
The reaction worked, and it had a strong preference for attaching an acetate to the carbon at position 2. That result left the phosphate hanging off position 3, like it normally is in the DNA and RNA used by existing life.
But that result wasn't the only thing this reaction changed. By altering the chemical environment near the phosphate, the resulting nucleotide became more reactive. Normally, these nucleotides will only react spontaneously to form chains a handful of nucleotides long. But with the acetate added, two of these short pieces would spontaneously link together, forming part of a longer RNA chain.
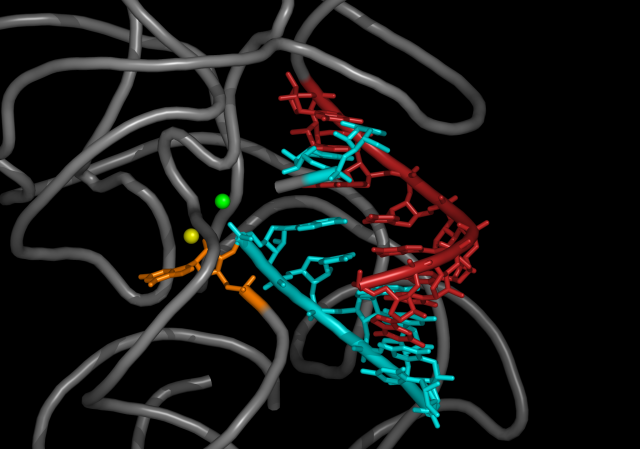
(This reaction required a third, short piece of RNA before it would occur. This third piece base-paired with both of the first two, lining them up so that the bits that underwent the reaction were in close proximity. Although this piece was supplied by the authors in this paper, in pre-biotic conditions, lots of random, short pieces of RNA would be around, so it isn't a problem from the perspective of whether this chemistry might work outside of the lab.)
There's nothing preventing this sort of reaction from occurring repeatedly, taking a large collection of short chains of nucleotides and gradually building up a significant piece of RNA from them. Since the starting material would have the individual nucleotides linked in a random order and lots of these reactions could occur in parallel, this situation could build up a large population of essentially random RNA molecules, and it's possible that some of those molecules could have catalytic activity.
Again, these findings don't mean that life definitively got its start through these acetylation reactions. But they do point the way toward a plausible path to large RNA molecules.
Nature Chemistry, 2013. DOI: 10.1038/NCHEM.1626 (About DOIs).
reader comments
31