Facts About Chromium

Known for its silver, shiny appearance, chromium is used to coat cars, stoves and other appliances to protect them from corrosion and to improve their looks. Chromium's high melting point and stable structure also make it useful in the textile and refractory industries. When combined with other elements, chromium makes vibrant colors and is used as a dye, which is what originally earned it its name from the Greek word chroma for "color."
It is naturally found in compounds in the earth's crust. However, consuming high levels of chromium in polluted drinking water or inhaling fumes of the heated element can cause ulcers, cancer and other health problems.
Just the facts
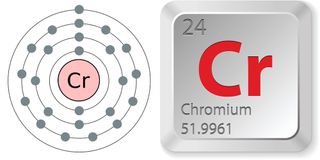
- Atomic number (number of protons in the nucleus): 24
- Atomic symbol (on the periodic table of elements): Cr
- Atomic weight (average mass of the atom): 51.9961
- Density: 4.13 ounces per cubic inch (7.15 grams per cubic cm)
- Phase at room temperature: solid
- Melting point: 3,465 degrees Fahrenheit (1,907 degrees Celsius)
- Boiling point: 4,840 F (2,671 C)
- Number of natural isotopes (atoms of the same element with a different number of neutrons): 4
- Most common isotope: Cr-52
Discovery of chromium
French chemist Louis Nicolas Vauquelin first isolated chromium from a bright red mineral called Siberian red lead, now known as lead chromate (PbCrO4) in 1797. According to John Emsley in his book, "Nature’s Building Blocks: An A-Z Guide to the Elements" (Oxford University Press, 1999), it took Vauquelin a year before he was able to precipitate out the lead and obtain pure chromium. He mixed the chromium in a variety of solutions and was intrigued by the many colors it produced, thereby naming the element after the Greek word chromameaning "color."
Sources of chromium
Chromium is the 21st most common element found in the Earth's crust, but it is not found in its free metal form. Instead, it is principally found in chromite ore, according to Robert E. Krebs in his book, "The History and Use of Our Earth’s Chemical Elements: A Reference Guide" (Greenwood Publishing Group, 2006). Chromite is mined in Zimbabwe, Russia, New Zealand, Turkey, Iran, Albania, Finland, the Philippines and Madagascar. About 20,000 tons of chromium metal are produced each year, and there are still about a billion tons of unexploited deposits in Greenland, Canada, and the United States, according to Emsley. Chromium metal is then obtained by heating the chromite ore in the presence of aluminum or silicon, according to the Jefferson Lab.
Properties of chromium
Chromium is a transition metal in Group 6 on the Periodic Table of Elements. In its pure form, chromium is a silvery, lustrous, hard metal that has a high polish, ideal for electroplating.
The most important chromium compounds are the chromates of sodium and potassium, the dichromates, and potassium and ammonium chrome alums, according to the Los Alamos National Laboratory. Chromium compounds are toxic and should be handled with care.
Chromium compounds are all vividly colored and are used as pigments — bright green, yellow, red and orange. Rubies are red because of chromium, and glass treated with chromium has an emerald green color, according to the Royal Society of Chemistry (RSC).
Used for protection and beauty
Using a technique called electroplating, a thin layer of chromium can coat metal and plastic objects, including car parts and household appliances, to give a shiny, attractive finish. For example, automotive designers use chrome rims and wheels to spruce up their cars. Chrome plating is not only used for looks; because the chromium forms a protective oxide layer on the surface, chrome-plated objects resist corrosion, according to Krebs.
Stainless steel is an alloy of iron and at least 10.5 percent chromium. It is highly resistant to corrosion. It is used in kitchen cutlery, appliances and cookware such as stainless steel pans and skillets.
About 90 percent of leather is chrome tanned, according to Emsley. During this process, chromium sulfate is used to treat animal skin and turn it into leather that is resistant to hot water that can cause degradation.
Kilns and furnaces use bricks made of chromite ore, which retains strength at high temperatures. The textile industry uses chromium ions to help adhere dyes to fabric. Chromium's high melting point, moderate thermal expansion and crystalline structure stability make it suitable for these purposes.
Dangerous in excess
Though its specific role in humans is unclear, studies have shown that chromium is an essential trace element that is found in RNA and helps the body to use glucose. Chromium is most concentrated in the placenta, and its presence in the body decreases with age.
A safe amount is about 1 milligram per day, according to the RSC. Foods such as brewer's yeast, wheat germ and kidney are rich in chromium. However, it is poisonous in excess.
In the 2000 film "Erin Brockovich," Julia Roberts portrays an environmental activist and legal clerk who leads a case against a gas and electric company for contaminating drinking water with chromium(VI), a toxic compound known to cause cancer, according to the U.S. Occupational Safety and Health Administration (OSHA). Though Brockovich won the case in real life and in the movie, scientists have criticized her lack of evidence showing that chromium(VI), and not other factors, was the cause of the numerous health issues including cancer in the community of Hinkley, Calif. However, several studies in the past few years have linked chromium(VI) in tap water to cancer and the EPA has set a drinking water concentration limit for the element to protect public health.
Excessive exposure to chromium can also cause chrome ulcers, particularly when working with it for months while chrome-plating or leather tanning, according to Emsley. Chrome ulcers are itchy and raw holes in the skin or the stomach. If dust containing chromium is inhaled, industrial workers are more prone to ulcers in the nasal cavity and lung cancer. Additionally, a 2017 study published in European Journal of Clinical Nutrition linked high levels of urinary chromium to cardiovascular disease and diabetes.
Additional resources
- Here is more info on chromium from the Los Alamos National Laboratory.
- Here is what the Jefferson Lab says about chromium.
- The Royal Society of Chemistry also weighs in about chromium.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.

